Abstract

Introduction Ibrutinib (IBR) is an oral, small-molecule irreversible inhibitor of Bruton's tyrosine kinase (BTK) approved for treatment-naïve and relapsed/refractory (R/R) pts with CLL at a dose of 420 mg/d. IBR binds covalently to the C481 residue of BTK in a 1:1 stoichiometric ratio. In a phase 1 trial of IBR in pts with R/R B-cell malignancies, no MTD was identified, and ≥95% BTK occupancy was observed at doses ≥2.5 mg/kg/d and correlated with clinical response (Advani, JCO 2013). IBR inhibits a number of other kinases at pharmacologically relevant concentrations (Honigberg, PNAS 2010), which may explain its off-target toxicities, e.g., atrial fibrillation (AF) and bleeding. We previously showed that BTK protein levels in CLL cells from patients on IBR decline over time (Cervantes-Gomez, Leukemia 2016). Therefore, we hypothesized that lower doses of IBR after 1 cycle at 420 mg/d would be sufficient to achieve ≥95% BTK occupancy and inhibit B-cell receptor (BCR) signaling in CLL cells, potentially leading to improved safety and cost savings while maintaining efficacy. Retrospective studies conducted at multiple centers in the US and UK have demonstrated that dose reduction of IBR does not compromise clinical outcomes (Mato, BJH 2017, UK CLL Forum, Haematologica 2016).
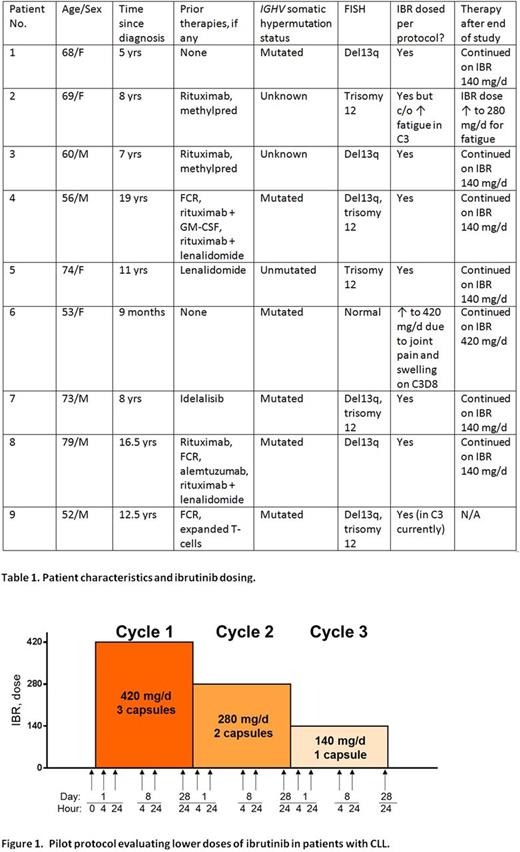
Methods We designed a pilot study (NCT02801578) in which pts with CLL and ALC ≥20 x 109/L received 420 mg/d, 280 mg/d and 140 mg/d of IBR in 3 consecutive 4-week cycles. Blood was obtained for measurement of IBR levels and various pharmacodynamic (PD) endpoints at 16 timepoints per pt (Figure 1). Response assessment was clinical; bone marrow examinations and imaging were not performed. After completion of the study, pts could continue on IBR (dosed according to their treating physician's discretion) or change therapy as clinically indicated.
Results Eleven pts were treated; 1 came off the study after 21 days of IBR (420 mg/d) in C1 and 1 after 21 days of IBR in C1 (420 mg/d) and 5 days of IBR (280 mg/d) in C2 (AAA repair and intolerance to IBR, respectively) and are not evaluable. One pt remains on study. Pharmacokinetic (PK) and PD analyses of blood samples from the other 8 pts have been completed or are ongoing. Baseline pt characteristics and clinical outcomes are summarized in Table 1. Updated results will be presented at the meeting.
IBR levels reached an average (avg) of 210 nM on C1D1 at 4h, 240 nM on C2D1, and 63 nM on C3D1 at 4h (n= 8). In all 3 cycles, by 24h after the D1 dose, avg IBR plasma concentrations fell to 25, 22, and 8 nM, respectively. Similarly, avg IBR levels in circulating CLL cells reached 113, 242, and 44 nM in cycles 1-3 on D1 at 4h, respectively (n=5).
BTK occupancy was measured by Pharmacyclics using a proprietary high affinity fluorescence probe. In all 8 pts analyzed thus far, an avg ≥98% of target BTK was covalently bound by IBR.
BTK phosphorylation at Tyr223 (measured by protein immunoblot analysis) was decreased at all 3 doses, and at 140mg/d of IBR in cycle 3, the levels were 40-50% of control, similar to cycles 1 and 2. Likewise, there was a decline in total BTK levels. PLCgamma2 phosphorylation at Tyr1217 was also reduced over the entire duration of IBR treatment. As reported at the 420mg/d dose, there was a notable increase in pro-apoptotic Bim, while levels of Bcl-2 remained constant. In contrast, there was a decline in Mcl-1 levels. In parallel, there was a decline in S6 (Ser235/236) phosphorylation in CLL cells during therapy. There was also evidence of NF-kappaB pathway modulation, and an increase in IkappaBalpha protein levels, coupled with a decrease in NFAT1 and SHP-1 levels. Further work is ongoing to see if the mRNA levels of the BCR downstream targets are also reduced during therapy with IBR.
IBR impairs platelet function. We evaluated platelet functionality using light transmission aggregometry. Data in 2 pts suggest that the lower doses of IBR in cycles 2 and 3 led to reduced inhibition of ristocetin-induced aggregation.
Conclusions Taken together, these data suggest that IBR doses as low as 140 mg/d are sufficient to fully occupy BTK and abrogate kinase function. As off-target effects of IBR are not thought to contribute to clinical efficacy in CLL, exploration of lower doses is warranted to reduce the incidence of troublesome side effects such as AF and bleeding, while preserving efficacy. Accordingly, a larger, more definitive trial with clinical endpoints of IBR at a dose of 140 mg/d following 2 cycles at 420 and 280 mg/d is planned.
Thompson: Pharmacyclics: Honoraria, Membership on an entity's Board of Directors or advisory committees. Jain: BMS: Research Funding; Abbvie: Research Funding; ADC Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Research Funding; Incyte: Research Funding; Novimmune: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Research Funding; Verastem: Research Funding; Adaptive Biotechnologies: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Wierda: The University of Texas MD Anderson Cancer Center: Employment; Acerta: Research Funding; Celgene: Consultancy, Honoraria; Gilead: Consultancy, Honoraria, Research Funding; Sanofi: Consultancy, Honoraria; Emergent: Consultancy, Honoraria, Research Funding; Merck: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria, Research Funding; Kite: Research Funding; Pharmacyclics: Consultancy, Honoraria, Research Funding; Genentech/Roche: Consultancy, Honoraria, Research Funding; Karyopharm: Research Funding; Janssen: Research Funding; Juno: Research Funding; GSK/Novartis: Consultancy, Honoraria, Research Funding; Genzyme: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal